METAL NON METAL

Physical Properties of Metals
- Metallic Lustre: Metals have a shiny surface in their pure state, known as metallic lustre (e.g., gold, silver, platinum).
- Physical State: Metals are generally solids at room temperature.
- Exception: Mercury is the only metal that is liquid at room temperature.
- Hardness: Most metals are hard, like iron and copper.
- Exception: Metals like lithium, sodium, and potassium are soft and can be cut with a knife.
- Ductility: This refers to the ability of a metal to be drawn into thin wires. Metals are generally ductile, with gold being the most ductile.
- Malleability: This is the ability of a metal to be beaten into thin sheets. Gold and silver are the most malleable metals.
- Electrical Conductivity: This is the ability to conduct electricity. Most metals are good conductors. Silver is the best conductor, followed by copper, gold, aluminium, and tungsten.
- Electrical Conductivity: Metals conduct electricity due to free electrons.
- Exception: Mercury is a poor conductor, while lead is almost non-conducting.
- Thermal Conductivity: Metals are good heat conductors (e.g., copper, silver).
- Aluminum and copper are used in cookware for this reason.
- Exception: Lead and mercury are poor heat conductors.
- Melting and Boiling Points: Metals usually have high melting and boiling points.
- Exception: Gallium and caesium have low melting points and can melt in the hand.
- Sonority: Metals produce sound when struck and are called sonorous. This property makes them suitable for uses like school bells.
Chemical Properties of Metals
- Reaction with Oxygen:
- Metals react with oxygen to form metal oxides.
- Example:
4Al (s)+3O2 (g)→2Al2O3 (s)
This equation shows aluminium reacting with oxygen to form aluminium oxide.
Metal Oxides
- Basic Nature:
- Metal oxides are generally basic.
2. Amphoteric Oxides:
- Some metal oxides like aluminum oxide and zinc oxide can act as both acids and bases, making them amphoteric.
- Example Reactions:
Al2O3 (s)+6HCl (aq)→2AlCl3 (aq)+3H2O (l)
Al2O3 (s)+2NaOH (aq)→2NaAlO2 (aq)+H2O (l)Al2O3 (s)
3. Solubility and Alkalis:
- Metallic oxides are generally insoluble in water, but some form hydroxides (alkalis) when they dissolve.
- Examples:
Na2O (s)+H2O (l)→2NaOH (aq)
K2O (s)+H2O (l)→2KOH (aq)K2O (s)+H2O (l)→2KOH (aq)
Note
- Alkalis are water-soluble bases that turn red litmus blue.
Order of Reactivity with Oxygen
- Highly Reactive Metals:
- Sodium (Na) and Potassium (K): React vigorously with oxygen, often catching fire. Stored in kerosene to prevent accidents.
- Moderately Reactive Metals:
- Magnesium (Mg) and Aluminium (Al): Require heating to burn in air; form a protective oxide layer.
- Zinc (Zn): Burns only with strong heating.
- Iron (Fe): Rods/blocks don’t burn easily, but iron filings burn vigorously in flame.
- Less Reactive Metals:
- Copper (Cu): Does not burn but forms black copper(II) oxide on heating.
- Silver (Ag) and Gold (Au): Do not react with oxygen, even at high temperatures.
Reactivity Order:
Na>Mg>Zn>Fe>Cu>Ag
Anodising
- Purpose: To form a thick oxide layer on aluminum for protection against further oxidation.
- Process:
- Aluminum naturally forms a thin oxide layer when exposed to air.
- Anodising enhances this layer by using an electric current.
- The aluminum article is used as the anode, with dilute H2SO4 (sulfuric acid) as the electrolyte.
- Oxygen gas is liberated at the anode, reacting with aluminum to thicken the oxide layer.
- Benefits:
- Provides a protective, durable finish.
- The oxide layer can be dyed for an attractive appearance.
Reaction of Metals with Water
- General Reaction:
- Metal + Water → Metal Oxide + Hydrogen Gas
- Metal Oxide + Water → Metal Hydroxide
2. Sodium (Na) and Potassium (K):
- React violently with cold water.The reactions are very exothermic.
- Potassium Reaction:
2K (s)+2H2O (l)→2KOH (aq)+H2 (g)
3. Sodium Reaction:
2Na (s)+2H2O (l)→2NaOH (aq)+H2 (g)
The heat produced can ignite the hydrogen gas, so these metals are stored in kerosene to prevent contact with air and water.
Reaction of Calcium with Water
- Reaction:
Ca (s)+2H2O (l)→Ca(OH)2 (aq)+H2 (g)
2. Characteristics:
- The reaction is less violent compared to sodium and potassium.
- The heat produced is not enough to ignite the hydrogen gas.
- Calcium floats on water because hydrogen gas bubbles stick to its surface.
Reaction of Magnesium with Water
- With Hot Water:
- Magnesium reacts with hot water to form magnesium hydroxide and hydrogen gas.
Mg (s)+2H2O (l)→Mg(OH)2 (aq)+H2 (g)
2. With Steam:
- Magnesium reacts with steam to produce magnesium oxide and hydrogen gas.
Mg (s)+H2O (g)→MgO (s)+H2 (g)
3. Characteristics:
- Magnesium does not react with cold water.
- It floats on water due to hydrogen gas bubbles adhering to its surface.
Reaction with Steam
- Aluminum (Al), Iron (Fe), and Zinc (Zn):
- Do not react with cold or hot water.
- React with steam to form metal oxides and hydrogen gas.
2. Aluminum Reaction:
2Al (s)+3H2O (g)→Al2O3 (s)+3H2 (g)
3. Iron Reaction:
3Fe (s)+4H2O (g)→Fe3O4 (s)+4H2 (g)
No Reaction with Water
- Lead (Pb), Copper (Cu), Silver (Ag), and Gold (Au):
- Do not react with water at all.
Reactivity Order Towards Water
K>Na>Ca>Mg>Al>Fe>Pb>Cu>Ag>Au
- Reaction of Metals with Dilute HCl
- General Reaction:
- Metal + Dilute Acid → Salt + Hydrogen Gas
- Examples:
- Zinc (Zn):
Zn (s)+2HCl (aq)→ZnCl2 (aq)+H2 (g)
- Iron (Fe):
Fe (s)+2HCl (aq)→FeCl2 (aq)+H2 (g)
Reactivity Order:
- The rate of hydrogen gas formation decreases in the order:
Mg>Al>Zn>FeMg>Al>Zn>Fe
- This order indicates the decreasing reactivity of these metals with dilute HCl.
- Note:
- Copper (Cu) does not react with dilute HCl.
Aqua-regia (Latin for “Royal Water”)
- Composition:
- A mixture of concentrated hydrochloric acid and concentrated nitric acid in a ratio of 3:1.
- Properties:
- Can dissolve gold and platinum, which neither acid can do alone.
- Highly corrosive and fuming.
- Uses:
- One of the few reagents capable of dissolving noble metals like gold and platinum.
Reaction of Metals with Solutions of Other Metal Salts
- Principle:
- More reactive metals can displace less reactive metals from their compounds in solution or molten form.
- Application:
- This principle is used in displacement reactions to extract or purify metals.
Displacement Reaction
- Concept:
- If metal A is more reactive than metal B, it can displace B from its salt solution.
2. General Reaction:
Metal A + Salt Solution of B → Salt Solution of A + Metal B
3. Example:
- Copper (Cu) displacing silver (Ag) from silver nitrate:
Cu (s)+2AgNO3 (aq)→Cu(NO3)2 (aq)+2Ag (s)
Type of Reaction:
- This is called a displacement reaction.
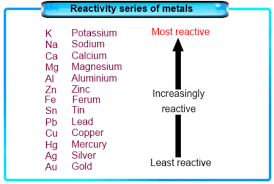
Reactivity Series of Metals
- Definition:
- A list of metals arranged in order of decreasing reactivity.
- Based on their tendency to lose electrons and form cations.
2. Characteristics:
- More reactive metals have a greater tendency to lose electrons.
3. Classification:
- Most Reactive Metals:
- Placed above hydrogen.
- Examples: Potassium (K), Sodium (Na), Calcium (Ca).
- Least Reactive Metals:
- Placed below hydrogen.
- Examples: Noble metals like gold (Au) and platinum (Pt).
Hydrogen in the Reactivity Series
- Properties:
- Hydrogen has non-metallic properties.
- Reason for Inclusion:
- Due to its electropositive nature, hydrogen is placed in the reactivity series.
NON – METALS
Physical Properties of Non-Metals
- Lustre:
- Non-metals generally do not have lustre.
- Exception: Diamond, graphite (allotropic forms of carbon), and iodine have lustre.
- Physical State:
- Non-metals are either solids or gases.
- Exception: Bromine is liquid at room temperature.
- Softness:
- Most non-metals are soft when solid.
- Exception: Diamond is the hardest known substance.
- Malleability and Ductility:
- Non-metals are neither malleable nor ductile.
- Brittleness:
- Non-metals are brittle. They break into pieces when hammered.
- Electrical and Thermal Conductivity:
- Non-metals are poor conductors of heat and electricity.
- Exception: Graphite is a good conductor of electricity.
- Melting and Boiling Points:
- Generally, non-metals have low melting and boiling points.
- Solid non-metals have higher boiling points compared to gases. Diamond has a very high melting and boiling point.
Chemical Properties of Non-Metals
- Reactivity with Water and Acids:
- Non-metals do not react with water, steam, or dilute acids to produce hydrogen gas.
- Reaction with Concentrated Acids:
- When heated, non-metals readily form oxides or salts with concentrated acids.
- Examples:
- Sulfur reacts with concentrated sulfuric acid:
S(s)+2H2SO4 (conc.)→Heat 3SO2 (g)+2H2O(l)
Sulfur reacts with concentrated nitric acid:
S(s)+6HNO3 (conc.)→HeatH2SO4 (aq)+6NO2 (g)+2H2O(aq)
NO2 is released as a reddish-brown vapor.
Displacement Reaction of Non-Metals
- Example:
- Chlorine displaces bromine in sodium bromide:
Cl2 (g)+2NaBr (l)→2NaCl (l)+Br2 (g)
Chlorine gas reacts with liquid sodium bromide to produce liquid sodium chloride and bromine gas.
- Note:
- Most non-metals produce acidic oxides when dissolved in water.
Ionic Bond Formation
- Reactivity of Elements:
- Elements aim for a completely filled valence shell (2 or 8 electrons).
- Tendencies:
- Metals tend to lose electrons to form cations (positive ions).
- Non-metals tend to gain electrons to form anions (negative ions).
- Ionic Bonds:
- Formed by the complete transfer of electrons from metals to non-metals.
- This results in ionic or electrovalent compounds.
- Example:
- Formation of Sodium Chloride (NaCl):
- Sodium (Na) is a metal with electronic configuration 2,8,1
- To achieve a stable configuration, sodium loses one electron from its outer shell, forming a cation (Na+).
- The sodium atom then has 11 protons and 10 electrons.
- Formation of Sodium Chloride (NaCl):
- Sodium Chloride (NaCl):
- Chlorine:
- Electronic configuration: 2,8,7.
- Gains one electron to complete its octet, forming a chloride ion (Cl−).
- Sodium:
- Loses one electron to form a sodium ion (Na+).
- Ionic Bond:
- Na+ and Cl− attract each other through electrostatic forces, forming NaCl.
- NaCl does not exist as discrete molecules but as aggregates of ions.
- Chlorine:
- Magnesium Chloride (MgCl2):
- Magnesium:
- Electronic configuration: 2,8,2.
- Loses two electrons to form a magnesium ion (Mg2+)
- Chlorine:
- Gains one electron each, forming two chloride ions (Cl−).
- Ionic Bond:
- Mg2 + attracts two Cl− ions, forming MgCl2.
- Magnesium:
Properties of Ionic Compounds
- Physical Nature:
- Ionic compounds are hard crystalline solids.
- They have strong forces of attraction between positive and negative ions.
- Generally brittle and break into pieces when pressure is applied.
- Melting and Boiling Points:
- Ionic compounds have high melting and boiling points.
- A large amount of energy is required to break the strong inter-ionic attractions.
- Solubility:
- Ionic (electrovalent) compounds are soluble in water.
- Insoluble in organic solvents like kerosene, benzene, ether, and petrol.
Conduction of Electricity:
- Good conductors of electricity in molten form or in aqueous solution.In solution or molten form, ions move freely and conduct electricity.
- Do not conduct electricity in solid form due to the rigid structure preventing ion movement.
Occurrence of Metals
- Sources:
- The earth’s crust is a major source of metals.
- Sea water contains soluble salts like sodium chloride and magnesium chloride.
- Minerals and Ores:
- Elements or compounds that occur naturally in the earth’s crust are called minerals.
- Minerals from which metals can be profitably extracted are called ores.
Extraction of Metals: Metallurgy
- Importance:
- Metals are crucial in daily life.
- Found in the earth’s crust in free state or as compounds (often oxides).
- Extraction Process:
- The process of obtaining pure metal from ore is called extraction of metals or metallurgy.
- The method depends on the nature of the ore, impurities, and metal to be extracted.
Extraction of Pure Metals
- Concentration or Enrichment of Ore:
- Initial step to remove impurities and concentrate the ore.
- Classification by Reactivity:
- Metals of High Reactivity:
- Extraction by electrolysis of molten ore to obtain pure metal.
- Metals of Medium Reactivity:
- Carbonate Ore: Undergoes calcination to form metal oxide.
- Sulphide Ore: Undergoes roasting to form metal oxide.
- Metals of Low Reactivity:
- Sulphide Ore: Undergoes roasting to form metal.
- Metals of High Reactivity:
- Reduction to Metal:
- Metal oxides are reduced to obtain the metal.
- Purification of Metal:
- Final refining to achieve pure metal.
Common Steps in Metallurgical Operations
Refining of Impure Metal: To achieve purity.
Enrichment or Concentration of Ore: To increase the metal content.
Extraction from Concentrated Ores: To obtain crude metal.