METAL NON-METAL MINDMAP
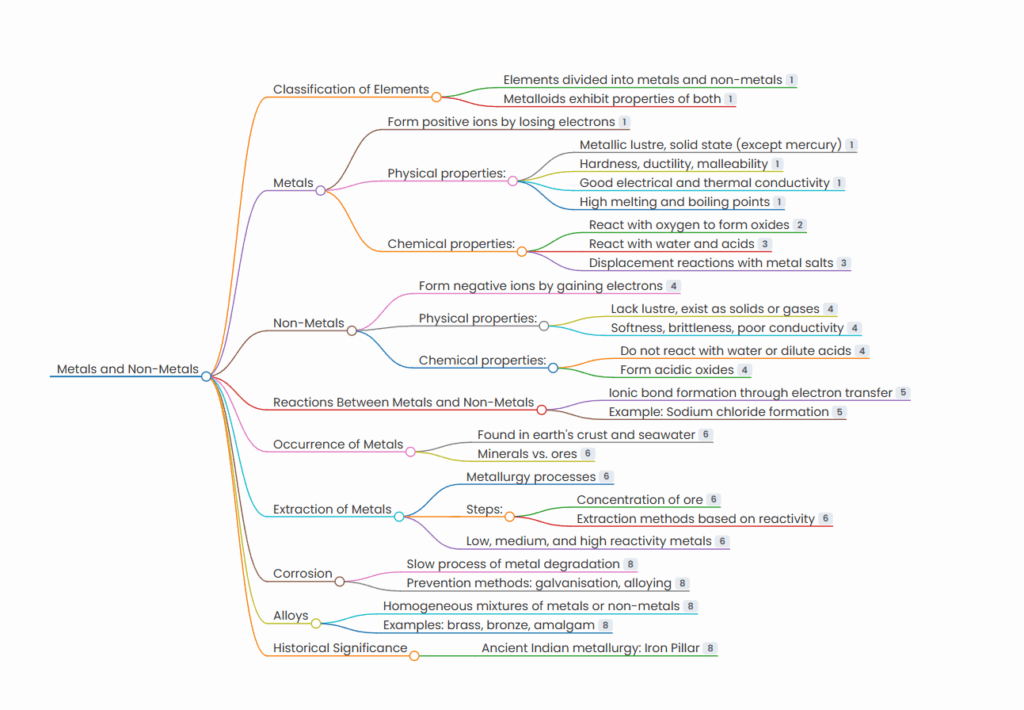
Classification of Elements
- Elements divided into metals and non-metals
- Metalloids exhibit properties of both
Metals
- Form positive ions by losing electrons
- Physical properties:
- Metallic lustre, solid state (except mercury)
- Hardness, ductility, malleability
- Good electrical and thermal conductivity
- High melting and boiling points
- Chemical properties:
- React with oxygen to form oxides
- React with water and acids
- Displacement reactions with metal salts
Non-Metals
- Form negative ions by gaining electrons
- Physical properties:
- Lack lustre, exist as solids or gases
- Softness, brittleness, poor conductivity
- Chemical properties:
- Do not react with water or dilute acids
- Form acidic oxides
Reactions Between Metals and Non-Metals
- Ionic bond formation through electron transfer
- Example: Sodium chloride formation
Occurrence of Metals
- Found in earth’s crust and seawater
- Minerals vs. ores
Extraction of Metals
- Metallurgy processes
- Steps:
- Concentration of ore
- Extraction methods based on reactivity
- Low, medium, and high reactivity metals
Corrosion
- Slow process of metal degradation
- Prevention methods: galvanisation, alloying
Alloys
- Homogeneous mixtures of metals or non-metals
- Examples: brass, bronze, amalgam
Historical Significance
- Ancient Indian metallurgy: Iron Pillar