ACID BASE AND SALT
Acids
- Definition: Chemical substances with a sour taste that turn blue litmus red.
Examples: Common fruits like unripe mango, lemon, and tamarind contain acids. Commonly used acids include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃).
Naturally Occurring Acids
- Vinegar: Acetic acid
- Orange and Lemon: Citric acid
- Tamarind: Tartaric acid
- Tomato: Oxalic acid
- Curd: Lactic acid
- Ant’s sting and nettle’s sting: Methanoic acid
Chemical Properties of Acids
Reaction with Metals:
- Acids like dilute HCl react with metals like zinc (Zn) and iron (Fe) to produce salt and hydrogen gas (H₂).
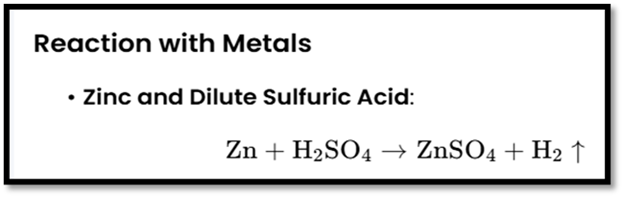
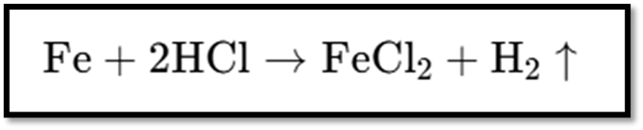
Test for Hydrogen Gas:
- Passing hydrogen gas through soap solution and bringing a burning splinter near the bubble. A pop sound indicates hydrogen.
Reaction with Metal Carbonates:
- Acids react with metal carbonates to produce salt, water, and carbon dioxide (CO₂) with effervescence.
- Example: Calcium carbonate reacts with hydrochloric acid to form calcium chloride, water, and carbon dioxide.

Test for Carbon Dioxide:
- Passing CO₂ through lime water turns it milky due to calcium carbonate formation.
- Excess CO₂ makes the solution clear by forming soluble calcium bicarbonate.
Initial Reaction
- Formation of Calcium Carbonate (Milky Solution):
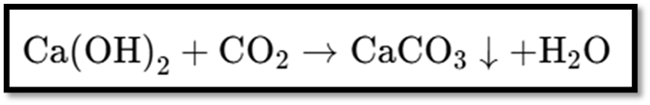
Reaction with Excess CO₂
- Formation of Soluble Calcium Bicarbonate (Clear Solution):
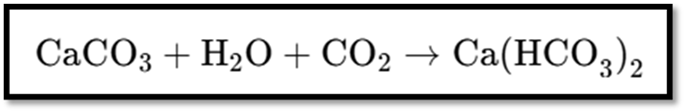
These equations describe how the solution changes from milky to clear with excess carbon dioxide.
Bases
- Definition: Chemical substances that are bitter, soapy to touch, and turn red litmus blue.
- Examples: Sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)₂).
Chemical Properties of Bases
- Reaction with Metals:
- Strong bases react with active metals to produce hydrogen gas.
- Example:
Reaction with Non-metallic Oxides:
- Bases react with non-metallic oxides to produce salt and water.
- Example:
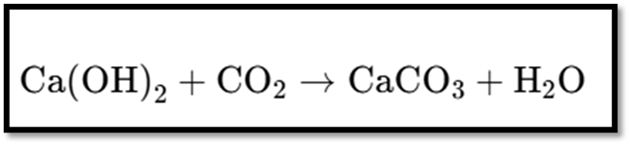
Effect of Dilution on an Acid or Base
- Dilution involves mixing acid or base with water, decreasing ion concentration.
- During dilution, add acid to water slowly to avoid heat generation and splashing.
Common Properties of Acids and Bases
- Acids: In water, give H⁺ ions.
- Bases: In water, give OH⁻ ions.
Reaction between Acids and Bases (Neutralization)
- Acids react with bases to produce salt and water.
- General Equation:
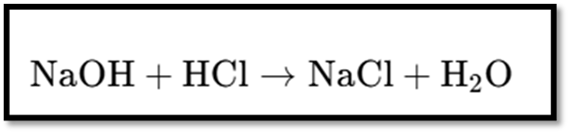
Indicators
- Purpose: Substances that change color or odor to indicate the presence of an acid or base.
Types of Indicators
- Natural Indicators:
- Obtained from natural sources.
- Examples:
- Litmus: Purple to red in acid, blue in base.
- Red Cabbage: Red in acid, green in base.
- Turmeric: Yellow in acid, reddish-brown in base.
- Flower of Hydrangea: Blue in acid, pink in base.
- Synthetic Indicators:
- Manufactured in laboratories.
- Examples:
- Phenolphthalein: Colorless in acid, pink in base.
- Methyl Orange: Red in acid, yellow in base.
- Universal Indicators:
- Mixture of indicators showing different colors at different pH values.
- Olfactory Indicators:
- Change in odor in acidic or basic medium.
- Examples: Vanilla, clove, onion.
Strength of Acids and Bases
- Determined by the number of H+H+ or OH−OH− ions.
- More H+H+ ions = stronger acid.
- More OH−OH− ions = stronger base.
The pH Scale
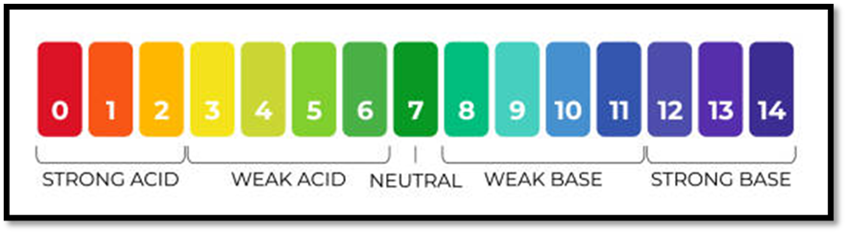
- Measures hydrogen ion concentration.
- Scale ranges from 0 (very acidic) to 14 (very basic).
- Neutral: pH 7
- Acidic: pH < 7
- Basic: pH > 7
- Measurement: pH meter or universal indicator paper.
This information helps in understanding how substances are classified based on their acid or base nature and how they interact with indicators.
Importance of pH in Everyday Life
- Plants and Animals:
- pH range for survival: 7.0 to 7.8.
- Acid rain (pH < 5.6) lowers the pH of rivers, affecting aquatic life.
- Soil pH:
- Specific pH ranges are needed for plant growth.
- Knowing soil pH helps in selecting fertilizers.
- Digestive System:
- Stomach produces hydrochloric acid (HCl) for digestion.
- Excess acid causes irritation; antacids like magnesium hydroxide neutralize it.
- Tooth Decay:
- Tooth enamel made of calcium hydroxyapatite.
- pH below 5.5 leads to decay; bacteria produce acids from food.
- Self Defence in Nature:
- Some insects inject acid, causing pain.
- Baking soda neutralizes stings.
Salts
- Production: Formed by neutralization of acids and bases.
- Types:
- Salts of strong acids and bases are neutral (pH = 7).
- Salts of strong acid and weak base are acidic (pH < 7).
- Salts of weak acid and strong base are basic (pH > 7).
I. Common Salt (Sodium Chloride, NaCl)
- Preparation:
- From hydrochloric acid and sodium hydroxide.
- Obtained from seawater and rock salt.
- Uses:
- Cooking.
- Raw material for sodium hydroxide, baking soda, etc.
II. Caustic Soda
- Chemical Name: Sodium hydroxide
- Chemical Formula: NaOH
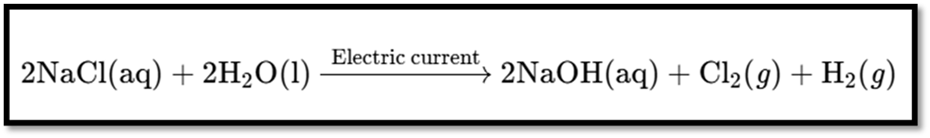
Preparation
- Produced by the chlor-alkali process.
Reaction:
Uses
- Hydrogen: Used in fuels and fertilizers.
- Chlorine: Used in water treatment, disinfectants, and PVC.
- Sodium Hydroxide: Used for cleaning, paper making, and synthetic fibers.
- Chlorine and Hydrogen: Raw materials for hydrochloric acid and bleach.
III. Bleaching Powder
- Chemical Name: Calcium oxychloride
- Chemical Formula: CaOCl₂
Preparation
- Produced by reacting chlorine with dry slaked lime.
Reaction:
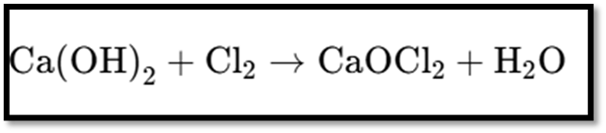
Uses
- Bleaching textiles and paper.
- Disinfecting water.
- Oxidizing agent in industries.
IV. Baking Soda
- Chemical Name: Sodium hydrogen carbonate
- Chemical Formula: NaHCO₃
Preparation
- Produced from sodium chloride.
Reaction:
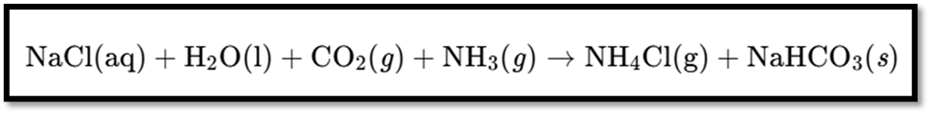
Baking Soda
- Chemical Name: Sodium hydrogen carbonate
- Chemical Formula: NaHCO₃
Properties
- Mild, non-corrosive base.
- Major component of baking powder.
Uses
Baking: Makes food soft and spongy.
Reaction:
- Antacid: Neutralizes stomach acid.
- Fire Extinguishers: Used in soda-acid type.
- Preservative for Milk: Neutralizes lactic acid.
Washing Soda
- Chemical Name: Sodium carbonate decahydrate
- Chemical Formula: Na₂CO₃·10H₂O
Properties
- White crystalline solid.
- Alkaline solution.
- Removes dirt and grease.
Uses
- Industries: Glass, soap, and paper.
- Manufacture: Used for making borax.
- Water Softening: Removes permanent hardness.
- Cleaning Agent: Used in laundry and housekeeping.
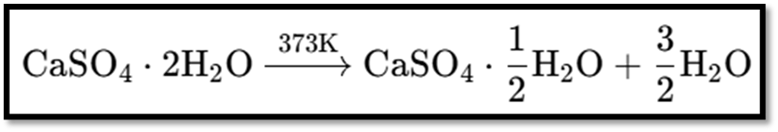
Plaster of Paris
- Chemical Name: Calcium sulphate hemihydrate
- Chemical Formula: CaSO₄·1221H₂O
Preparation
- Obtained by heating gypsum.
- Reaction:
Properties
- White powder.
- Hardens when mixed with water.
Uses
- Construction: Forms a hard solid mass.
- Art and Craft: Used for making sculptures and casts.
Uses of Plaster of Paris
- Medical: Used for setting fractured bones.
- Art and Decor: Making toys, decorative pieces, and ceiling designs.
Water of Crystallization
- Definition: Water molecules that are part of the crystalline structure of some salts.
- Hydrated Salts: Salts containing fixed numbers of water molecules.
- Example: Copper sulphate pentahydrate (CuSO₄·5H₂O).
- Example: Gypsum (CaSO₄·2H₂O).
- Plaster of Paris: Contains 1221 molecule of water of crystallization.
Action of Heat on Hydrated Salts
Heating: Removes water of crystallization, turning them into anhydrous salts.
- Example: