CHEMICAL REACTION AND EQUATION

Chemical Reactions
- Definition:
A process where substances (reactants) are transformed into new substances (products) with different properties.
- Examples:
Rusting of iron, digestion of food, fermentation.
Characteristics of Chemical Reactions
- Change in state
- Change in color
- Evolution of gas
- Change in temperature
- Formation of a precipitate
Chemical Equations
- Purpose: Simplifies the representation of chemical reactions.
- Word Equation
Example:
Methane + Oxygen → Carbon dioxide + Water
Writing a Chemical Equation
- Reactants: Written on the left with a plus sign (+).
- Products: Written on the right with a plus sign (+).
- Arrow (→): Separates reactants from products; indicates the direction of the reaction.
Example
Chemical Equation:
The image shows the chemical equation for the combustion of methane. Here’s the equation:
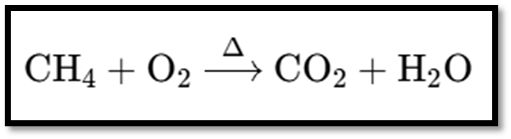
Explanation
- Reactants:
- CH44: Methane.
- O22: Oxygen.
- Products:
- CO22: Carbon dioxide.
- H22O: Water.
- ΔΔ: Indicates the application of heat.
This reaction depicts methane burning in the presence of oxygen, producing carbon dioxide, water, and releasing heat.
Balanced Chemical Equations
- Definition: A balanced equation has equal numbers of each type of atom on both sides, following the law of conservation of mass.
- Law of Conservation of Mass: Mass cannot be created or destroyed in a chemical reaction.
Balancing a Chemical Equation
- Method: Hit and trial method is used to balance equations by adjusting coefficients.
- Steps:
- Write the unbalanced equation.
- List the number of atoms of each element on both sides.
- Balance one element at a time using coefficients.
- Recheck atom counts to ensure balance.
Example Process
- Equation: Na + H₂O → NaOH + H₂
- Balancing Steps:
- Balance hydrogen by adjusting coefficients.
- Balance sodium next.
- Verify oxygen is balanced.
Making Chemical Equations Informative
- Physical States:
- Use (s) for solids, (l) for liquids, (g) for gases, (aq) for aqueous solutions.
- Precipitate Formation:
- Indicated by a downward arrow (↓).
- Gas Evolution:
- Indicated by an upward arrow (↑).
Additional Information in Equations
- Physical States:
Indicated by (s), (l), (g), (aq).
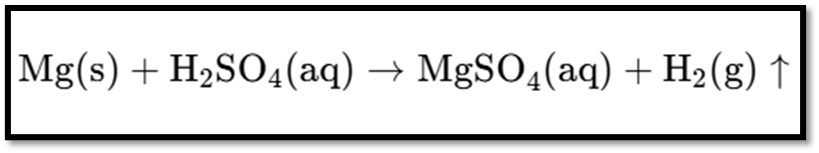
Explanation
- Reactants:
- Mg(s): Solid magnesium.
- H22SO44(aq): Aqueous sulfuric acid.
- Products:
- MgSO44(aq): Aqueous magnesium sulfate.
- H22(g): Hydrogen gas, indicated by the upward arrow (↑).
This reaction illustrates the formation of magnesium sulfate and the release of hydrogen gas when magnesium reacts with sulfuric acid.
- Reaction Conditions:
Temperature, pressure, catalysts noted above or below the arrow.
The image shows a chemical equation for the synthesis of methanol. Here’s the equation:
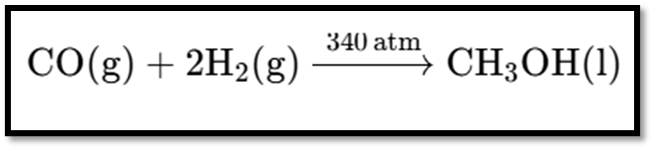
Explanation
- Reactants:
CO(g): Carbon monoxide gas.
2H2(g): Hydrogen gas.
Product:
CH3OH(l): Liquid methanol.
Conditions:
The reaction occurs at 340 atm pressure.
This reaction involves the use of high pressure to synthesize methanol from carbon monoxide and hydrogen.
- Heat Change:
Evolution or absorption shown as “+Heat” or “-Heat”.
Balancing Chemical Equations
The image shows the chemical equation for the combustion of carbon. Here’s the equation:
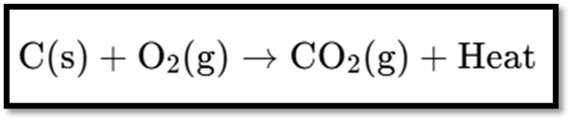
Explanation
- Reactants:
- C(s): Solid carbon.
- O22(g): Oxygen gas.
- Product:
- CO22(g): Carbon dioxide gas.
- Heat: The reaction releases heat, indicating it’s exothermic.
This reaction represents the burning of carbon in oxygen to produce carbon dioxide and release heat.
Types of Chemical Reactions
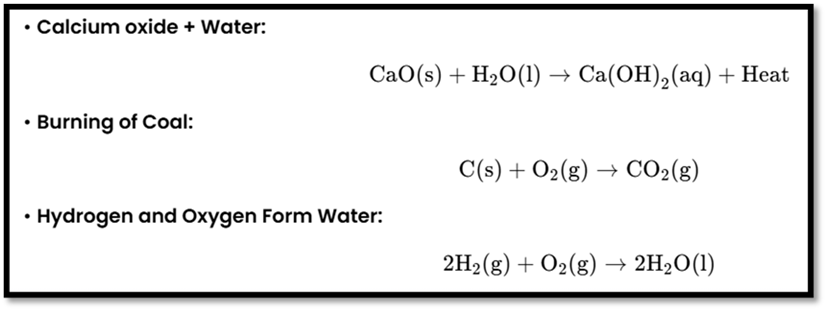
- Combination Reaction
Definition: Two or more reactants combine to form one product.
Examples:
- Decomposition Reaction
Definition: A single reactant breaks down into two or more products.
Types:
- Thermal Decomposition: Uses heat.
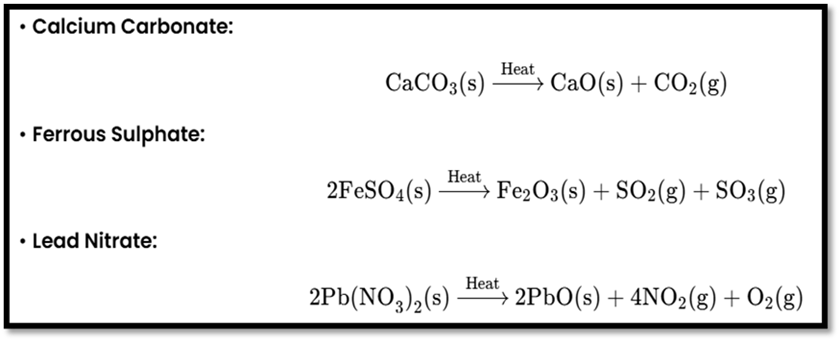
- Electrolysis: Uses electrical energy.
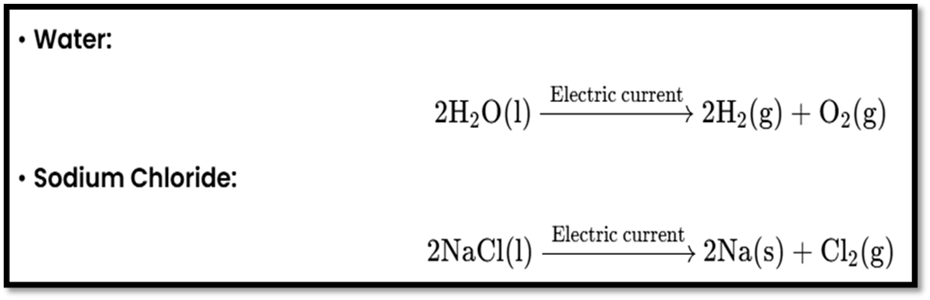
The image discusses types of reactions: photolysis, exothermic, endothermic, and displacement reactions. Here’s a summary:
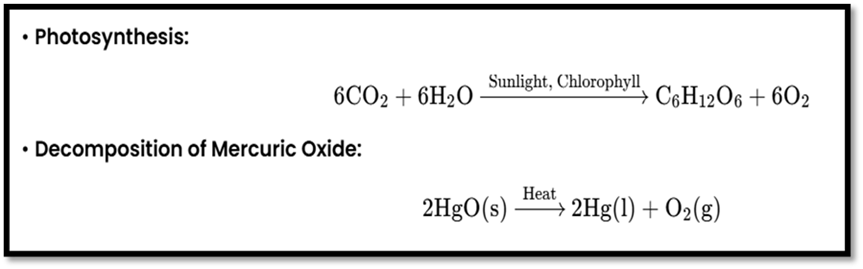
Photolysis or Photochemical Decomposition
Definition: Uses light energy to decompose compounds.
Examples:
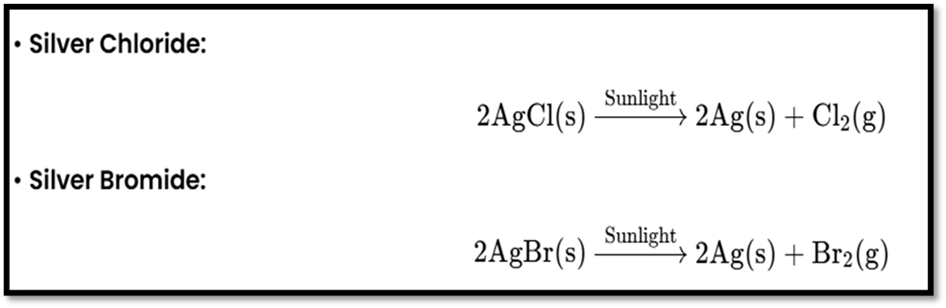
- Exothermic and Endothermic Reactions
Exothermic Reactions
Definition: Release heat or energy.
Examples:
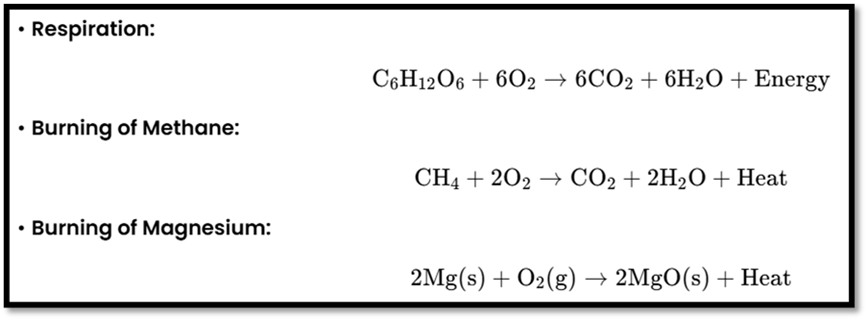
Endothermic Reactions
Definition: Absorb heat, energy, or light.
Examples:
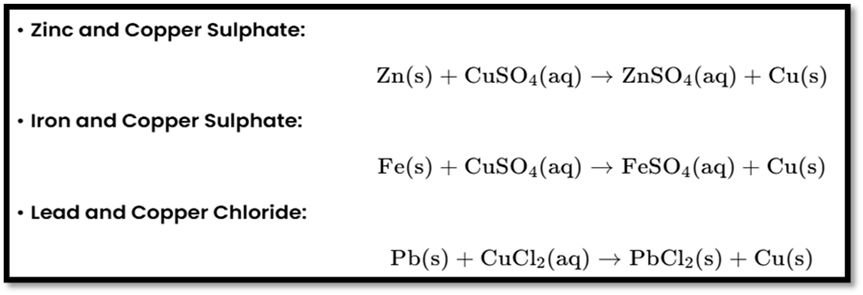
Displacement Reaction
Definition: A more reactive element displaces a less reactive one from its compound.
Examples:
Double Displacement Reaction
- Definition: Two different ions or groups of atoms in reactant molecules exchange places.
- Precipitation Reaction: Produces an insoluble substance (precipitate).
- Examples:
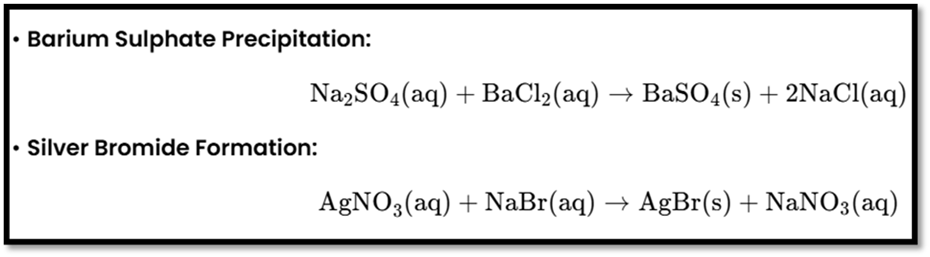
Oxidation and Reduction Reactions
Oxidation
Definition: Addition of oxygen or removal of hydrogen.
Examples:

Reduction
Definition: Removal of oxygen or addition of hydrogen.
Examples:

Redox Reactions
Definition: Oxidation and reduction occur simultaneously.
Examples:
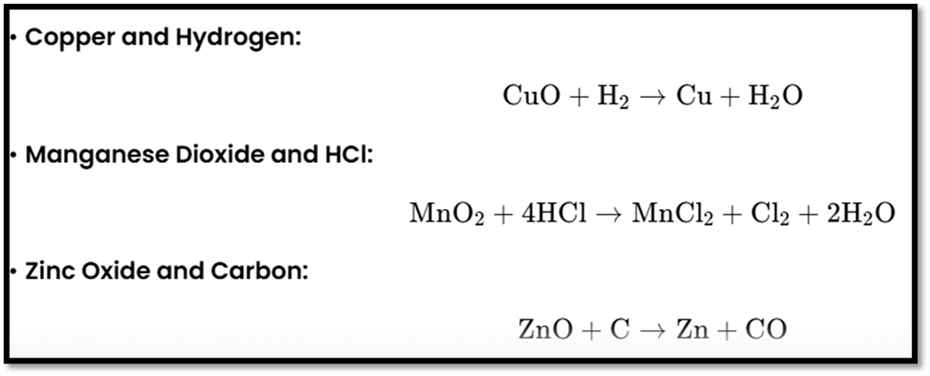
These reactions demonstrate the exchange of ions and simultaneous oxidation and reduction processes.
Corrosion Definition: The gradual destruction of metals due to reactions with air, water, and chemicals.
Example:
Rusting of Iron: Formation of reddish-brown iron oxide. Other Examples: Black coating on silver, green coating on copper.
Effects:
Wasteful process causing damage to metal structures like car bodies and bridges. Leads to significant financial costs for repairs and replacements.
Prevention:
Techniques include painting, galvanizing, and electroplating to protect metal surfaces. Rancidity
Definition: Slow oxidation of fats and oils in food, leading to off-flavours and odours.
Prevention Methods:
- Store food in air-tight containers. Refrigerate cooked food.
- Use nitrogen packaging for foods like chips.
- Add antioxidants to delay oxidation.
Understanding these processes and prevention methods helps in maintaining the integrity of metals and the quality of food.